Very Facile Production of Hydrogen Peroxide from Water under Sunlight
2020-0131-08

- researcher's name
-
about researcher NISHIDE, Hiroyuki Senior Research Professor (retired)
- affiliation
-
Faculty of Science and Engineering Waseda Research Institute for Science and Engineering
- keyword
-
background
● Hydrogen peroxide (H2O2) is widely used in a pulp and paper bleaching, wastewater treatment, chemical syntheses, etc. It is industrially manufactured using high pressure hydrogen, precious metallic catalyst, and organic solvents.
summary
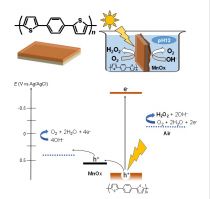
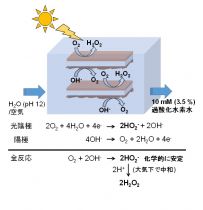
● We found that polythiophenes act as a rare, combined light-harvester and highly selective catalyst for the oxygen reduction to H2O2. Its combination with a conventional oxygen evolution catalyst in water (pH 12) achieved extremely high H2O2 production rate (>140 mg (H2O2) g-1 h-1).
predominance
● Extremely facile reaction vessel (Figure);
● Environmental friendly process (precious metal- free);
● Manufacturing on site.
● Environmental friendly process (precious metal- free);
● Manufacturing on site.
application/development
● The global market for H2O2 exceeds 3 million metric tons per year and is expected to reach twice within 10 years. This procedure provides a very facile and high-cost performance on site process, especially for yielding 5% H2O2 aqueous solution.
collaborative researchers
岡 弘樹
same researcher's seeds
-
 Organic “soft” secondary batteries
Organic “soft” secondary batteries
posted:
2020/02/06